Health
Landmark Decision: FDA Clears First OTC Birth Control Pill Enhancing Women’s Health Access

In an unprecedented advancement for women’s health, the U.S. Food and Drug Administration (FDA) has given the green light to the nation’s first over-the-counter (OTC) birth control pill. Named “Opill” (comprising norgestrel), this daily oral contraceptive has now been made accessible without the prerequisite of a prescription.
With this new permission, consumers can conveniently procure Opill across various outlets: from local drug stores, supermarkets, and corner stores to online platforms. The specifics surrounding its launch date and pricing are yet to be defined and will be unveiled by the manufacturer. Meanwhile, alternative formulations and strengths of oral contraceptives will remain prescription-dependent.
Patrizia Cavazzoni, M.D., at the helm of the FDA’s Center for Drug Evaluation and Research, expressed, “This milestone approval is transformative as it introduces a daily OTC oral contraceptive option for countless Americans. Employed as advised, such contraceptives are both secure and forecasted to surpass existing OTC contraceptive methodologies in curbing unexpected pregnancies.”
In light of statistics revealing that nearly half of the annual 6.1 million U.S. pregnancies are unexpected, the FDA’s hope with this OTC introduction is to dismantle access hindrances. This will empower individuals to acquire oral contraceptives without the immediate necessity for medical consultation. Unexpected pregnancies, which can culminate in undesired maternal and neonatal ramifications — from delayed prenatal attention to premature childbirth — underscore the significance of this move.
Opill’s birth control efficacy isn’t a recent discovery. The pill was initially sanctioned for prescribed utilization in 1973. Subsequently, HRA Pharma, now under the aegis of the Perrigo Company plc, sought to transition norgestrel from a prescription model to an OTC format. The FDA’s stipulations for such a shift mandate the drug to be demonstrably safe and effective for consumers, solely guided by OTC drug labeling, sans professional medical aid. Data indicates that users comprehended the Opill’s Drug Facts label proficiently, inferring their capability to judiciously administer the OTC drug.
However, a word of caution for consumers: punctuality is pivotal. Opill should be consumed daily at consistent intervals to retain its efficacy. The simultaneous consumption of other medications might compromise Opill’s efficiency, or the other drug, raising the likelihood of unintentional pregnancies.
Among the commonly observed side effects of Opill are sporadic bleeding, migraines, vertigo, nausea, heightened appetite, stomach discomfort, and menstrual cramps. Those with a history or current diagnosis of breast cancer or any cancer variant are advised to consult a physician prior to consumption. The simultaneous use of Opill with another hormonal contraceptive is strongly discouraged.
It’s crucial to discern that Opill isn’t a remedy for emergency contraception and is ineffective post-unprotected intercourse. Also, oral contraceptives aren’t shields against HIV, AIDS, or other sexually transmitted infections. The FDA remains firm in its recommendation of condoms as the protective measure against these diseases.
In essence, this groundbreaking FDA approval paves the way for monumental progress in women’s healthcare, ushering in a more user-friendly and accessible contraceptive avenue.
Health
Healthcare Developments and Services in the U.S. Virgin Islands

Significant strides have been made in healthcare services throughout the region, driven by robust reforms and initiatives designed to enhance the quality and accessibility of medical care for all residents. Here’s a comprehensive look at the key advancements shaping the healthcare landscape in the U.S. Virgin Islands:
Healthier Horizons Initiative
One of the most transformative efforts is Governor Albert Bryan Jr.’s “Healthier Horizons” initiative. This program aims to overhaul the healthcare system through three primary objectives: increasing access to healthcare, modernizing service delivery, and rebuilding healthcare infrastructure. The initiative encompasses 11 key elements, including behavioral health and developmental disabilities, health information exchange, telehealth, and telemedicine, as well as improvements to hospitals and health facilities.
Expansion of Telehealth Services
The Telehealth and Telemedicine Act, part of the Healthier Horizons initiative, seeks to bridge the gap in healthcare access by leveraging technology. A Telehealth Workgroup, formed in January, comprises members from both the public and private sectors, including healthcare providers and technology companies. This group is developing policies for Medicaid telehealth services and drafting legislation to establish a comprehensive telehealth framework.
Modernization of Healthcare Facilities
Ongoing efforts to modernize healthcare facilities are pivotal to the overall healthcare strategy. Key projects include the renovation and enhancement of the Gov. Juan F. Luis Hospital & Medical Center on St. Croix and the Roy Lester Schneider Hospital on St. Thomas. These facilities are being upgraded to provide state-of-the-art care and to better withstand future natural disasters, reflecting lessons learned from the hurricanes of 2017.
Health Information Exchange
A significant advancement in the digitalization of healthcare is the creation of a Health Information Exchange (HIE) for Medicaid patients. Funded by a $14.9 million grant from the Centers for Medicare and Medicaid Services, this exchange will enable healthcare providers to securely share patient information, thus improving the efficiency, safety, and cost-effectiveness of patient care.
Behavioral Health and Developmental Disabilities
Addressing mental health and developmental disabilities is a critical component of the Healthier Horizons initiative. Legislative measures, such as the Behavioral Health and Developmental Disabilities Act, are being introduced to provide better services and support for individuals affected by these conditions. This initiative aims to create a more inclusive and supportive healthcare environment for all residents.
Community Health Improvement Plan
The Community Health Improvement Plan focuses on preventive care and public health initiatives to enhance overall community health. This plan includes strategies for combating chronic diseases, improving maternal and child health, and increasing vaccination rates. These efforts are supported by public awareness campaigns and community health programs conducted by the Virgin Islands Department of Health.
Integration of Emergency Services
Improving emergency medical services is another priority under the Healthier Horizons initiative. Efforts are underway to integrate the Virgin Islands Fire Service with emergency medical services to ensure a more cohesive and efficient response to emergencies. This integration aims to enhance the overall emergency response capabilities across the islands.
Medicinal Cannabis Program
The introduction of a medicinal cannabis program marks a progressive step in healthcare reform. The Virgin Islands Cannabis Use Act is designed to regulate the use of medicinal cannabis, providing patients with access to alternative treatments for various medical conditions. This program is expected to complement traditional medical treatments and offer new avenues for patient care.
Housing and Healthcare Services
The V.I. Healthy Housing Initiative is a collaborative effort with local stakeholders to address housing and healthcare services for vulnerable populations, including behavioral health, youth rehabilitation, supportive housing, and elder care. This initiative aims to create a supportive infrastructure that addresses both housing and healthcare needs in an integrated manner.
Conclusion
The U.S. Virgin Islands is undergoing a significant transformation in its healthcare system, driven by comprehensive reforms and strategic initiatives. These efforts aim to provide residents with enhanced access to quality medical care, modernize healthcare delivery, and build a resilient healthcare infrastructure capable of meeting future challenges. Through initiatives like Healthier Horizons, telehealth services, and the modernization of healthcare facilities, the USVI is setting a new standard for healthcare in the region.
Health
Governor Bryan Pushes for Urgent Legislation to Address Medicaid Fund Shortfall

Governor Albert Bryan announced on Monday that the territory has exhausted its funds for the local Medicaid match, prompting Government House to submit draft legislation to the Senate seeking an additional $3 million for this purpose.
Despite the fiscal shortfall, Governor Bryan views the situation as a sign of increased healthcare access. “We’re having so many people access care,” he stated, highlighting that during the pandemic, nearly 40,000 individuals utilized Medicaid for services including braces, dental care, and various medical appointments. Furthermore, eased referral requirements have facilitated access to specialty care. “Before, you had to go to East End or Frederiksted Health Center for a referral; now, a regular doctor can refer you,” Bryan explained.
These expanded services and simplified processes have rapidly depleted the Medicaid matching funds. However, Governor Bryan does not foresee this as a recurring issue, predicting stabilization next year. He noted that the V.I. Department of Human Services has already reduced some services, which has led to a decrease in Medicaid enrollment.
The proposed $3 million allocation remains critical for Virgin Islanders. Governor Bryan emphasized its importance, pointing to the recent U.S. Department of Defense Innovative Readiness Training (IRT) Program health fair, where medics served over 2,500 people seeking no-cost healthcare services. Although 6,000 people applied, many were turned away due to limited resources. The governor stressed that healthcare costs for uninsured residents ultimately fall on the territory, whether through Medicaid or hospital services.
The effort to secure adequate Medicaid funding is ongoing. In 2019, Congresswoman Stacey Plaskett successfully obtained an additional $252 million for the territory in a fiscal year 2020 spending bill, raising the federal match from 55% to 83%. This increased match rate, initially set to expire in 2021, has been made permanent, ensuring the territory receives the highest possible Medicaid match in the U.S.
Health
Measles Outbreaks Prompt Vaccination Drive in U.S. Virgin Islands
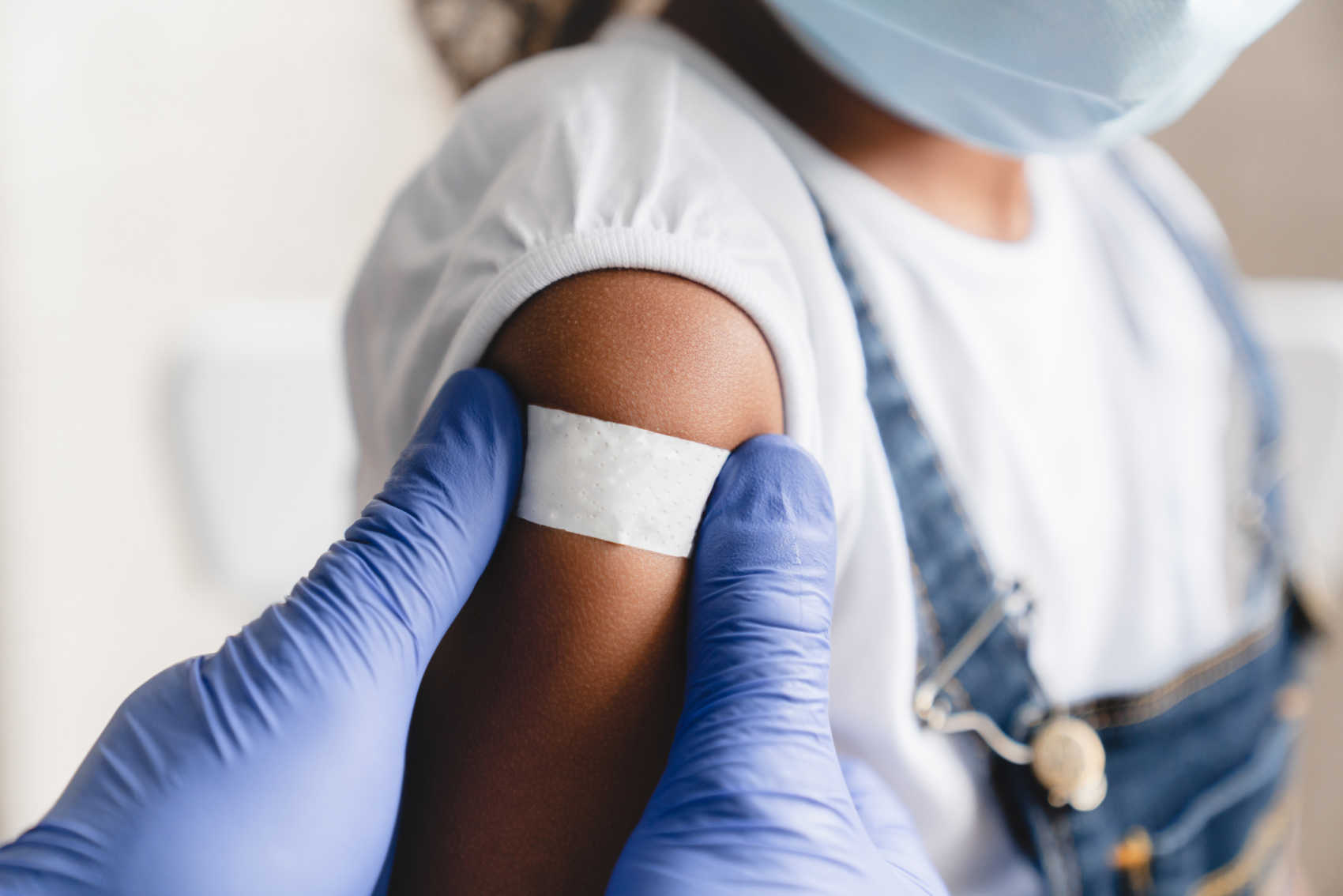
As measles outbreaks rise across the United States and the Caribbean, U.S. Virgin Islands Health Commissioner Justa Encarnacion is urging parents to prioritize their children’s vaccination schedules.
Commissioner Encarnacion voiced her concerns this week about the alarmingly low vaccination rates among children in the territory, emphasizing the imminent threat of measles. “We are very concerned about the low childhood vaccine rate in the Territory, especially with measles threatening the US,” she said. Currently, only 60 percent of USVI children are vaccinated, a situation worsened by an increasing number of parents seeking vaccination exemptions.
The urgency is underscored by the latest data from the Centers for Disease Control, which reports weekly on measles outbreaks. As of June 6, 2024, there have been 151 measles cases across 22 jurisdictions, including Arizona, California, Florida, and New York, with over half resulting in hospitalizations.
The infectiousness of measles is a significant concern for the V.I. Department of Health. “While 151 may seem like a low number, it is alarming because one person can infect nine to ten others,” Encarnacion explained. She also highlighted the risk of the disease spreading to the USVI and neighboring regions, noting that the Turks and Caicos Islands reported their first measles cases since 1991 this past May.
In response, the Department of Health launched the “Be Wise, Immunize” campaign earlier this year. This initiative aims to educate parents about the vital importance of vaccinations, stressing that immunization is the best defense against diseases like measles both in childhood and later in life.
The primary defense against measles is the measles, mumps, and rubella (MMR) vaccine, which provides long-lasting protection against all strains of the virus. Measles can lead to severe health complications, particularly in children under five, including pneumonia and encephalitis.
Measles is highly contagious, spreading through the air via coughs or sneezes from infected individuals. It remains active in the air or on surfaces for up to two hours. Symptoms typically appear seven to 14 days after exposure and include high fever, cough, runny nose, red watery eyes, and a characteristic rash.
Although declared eliminated in the United States in 2000, measles continues to persist globally and is often brought into the U.S. by unvaccinated travelers.
The VI Department of Health is urging parents to ensure their children are vaccinated and provides resources for scheduling immunization appointments at www.doh.vi.gov/immunization.
-

 Education1 year ago
Education1 year agoEducation Board Seeks Input on Schools Through Comprehensive Survey
-

 Education2 years ago
Education2 years agoCTE Board Enthusiastic About New Curriculum Standards, Yet Anxious Over Apprenticeship Support
-

 Crime2 years ago
Crime2 years agoRegistered Sex Offender Detained for Illegal Firearm Possession During Annual Surveillance Drive
-

 Development1 year ago
Development1 year agoCosts Surge as Donoe Estates Housing Project Resumes with New Contractor
-

 Videos3 years ago
Videos3 years ago2022 Gubernatorial Election: Voters Speak Out
-

 Videos3 years ago
Videos3 years agoGubernatorial Teams Celebrate St. Croix’s Bull & Bread Day
-

 Videos3 years ago
Videos3 years agoWakanda’s Female Might: A Dive into ‘Black Panther: Wakanda Forever’
-

 Crime2 years ago
Crime2 years agoSt. John’s Westin Resort Scene of Armed Robbery, Prompting Heightened Police Vigilance




